Diagnosis: Test passed
Whether it’s protective masks, respirators, or corona tests, the Covid pandemic has repeatedly demonstrated why medical goods must be of superior quality and available in large quantities - they save lives. PIA Automation demonstrates how speed and quality can be perfectly combined to create efficiency in the production of such goods through their valued partnership with a North American medical device manufacturer and the creation of multiple systems for rapid, portable PCR-tests production.
The medical device manufacturer had a vision to simplify the diagnosis of certain diseases so that they can be diagnosed quickly, anywhere, and as easily as possible. Since the founding of the company, their developers have been working on, among other things, detecting viral infections using portable, rapid PCR tests. PCR tests are the gold standard for early, reliable diagnosis of infectious diseases such as corona or influenza. However, the tests developed by this customer offer significant differences from test procedures in the laboratory: these are portable, barely larger than a cell phone, and are intuitive to operate. It also provides reliable test results within just 30 minutes.

"We have been working with this customer to develop systems that produce the only instrument-free rapid PCR test in a quick, precise and reliable manner to meet the demands of their customers," says David Kerbeck, Senior Account Manager at PIA Automation North America. "PIA has recently delivered their second system on which the PCR tests are manufactured in a fully automated way."
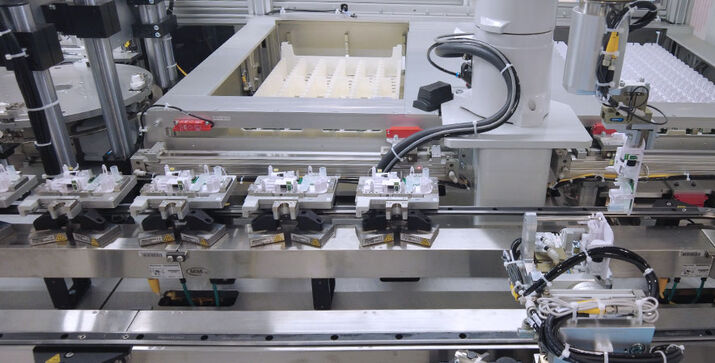
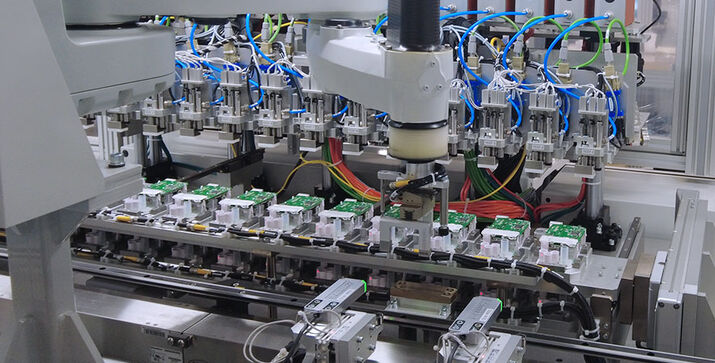
The performance data of the machine is impressive: it takes just five seconds to produce one PCR test, and twelve tests are produced each minute. A total of ten high-speed robots are installed in the system that includes the positioning of the test’s small, sensitive components as well as their assembly. Lyophilsed reagent beads of various sizes are also being fed through PIA Automation’s specially designed feeders. Also integrated in the line is an automatic function test, in which each test is for example analyzed for leaks. The PCR tests are produced at the customer’s facility in cleanroom class 8 according to DIN EN ISO 14644-1 and meet the highest hygiene requirements.
David Kerbeck has no doubt that the strong relationship between the two companies will continue, saying, "We are very excited about future revolutionary products from our customer and are proud that, with our production expertise, we can contribute to the global expansion of such innovative diagnostic procedures."